Basic Understanding of pH
Purpose:
To inform and acquaint you with the meaning of pH, and how it is applied in the use of cleaning compounds.
pH is a unit of measurement similar to meters, feet, and pounds. The concentration of ions containing hydrogen in a compound is measured and expressed as pH which means, percentage of hydrogen. Ions are the electrically charged particles of atoms. The charge is either positive (+) or negative (-).
Hydrogen ions are acidic in nature and are called acid ions. All acids contain hydrogen e.g. hydrochloric acid HCl, sulfuric acid H2SO4. HCl is hydrogen chloride, hydrochloric acid. H2SO4is hydrogen sulfate or sulfuric acid. Notice hydrogen is a part of both. The percentage of hydrogen determines acidity or alkalinity.
If a substance is alkali it contains alkali ions (OH). Hydroxides are alkaline in nature and are seen in such compounds as caustic soda or commonly referred to as lye (NaOH), or sodium hydroxide.
By knowing the above information, pH then is a measurement of the acidity or alkalinity of a solution.
In a solution containing water (H2O) there are present both alkaline as well as acidic ions. In a neutral cleaning solution, the alkaline ions and acidic ions are equal. In acidic solutions the acidic ions outnumber the alkaline ions. In an alkaline solution, the alkaline ions outnumber the acidic ions. Therefore it becomes possible by understanding this information to measure the pH of a compound.
The reason for understanding the importance of pH is critical in the proper selection of cleaners for a particular job.
What is really happening in cleaning is that one is attempting to "neutralize" the impact of the acidic or the alkaline ions. When a surface requires cleaning, the selection and use of the proper product results in the surface being cleaned or "neutralized".
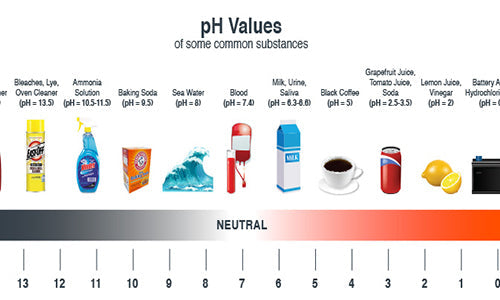
If one has a dusty floor which is otherwise clean, it would not be practical to use a heavy alkaline cleaner to remove the dust. One would respond to a problem of a dusty floor by either vacuum sweeping with a walk behind sweeper, like our warehouse sweeper, or dust-mopping it. In a number of ways all of us have interacted with this idea of neutralizing a surface or have had to clean something. We may not have been aware of pH but we nevertheless utilized the theory behind pH. Did you ever have an overactive acid problem in your stomach caused by overeating or eating the wrong foods? If so you probably took a commercial preparation intended to neutralize the acid in your stomach. And oh! What a relief it was. (Alka-Seltzer is an alkaline product). The purpose of the information herein is to help you thoroughly understand pH. The pH scale can be expresses as follows:

Most of the soils that we encounter are a combination of oils, dust, and dirt, and fall over the acid side of the scale. In order to clean a surface with acidic soiling, one would select a cleaner from the alkaline side of the pH scale, since an alkaline neutralizes an acid.
In cleaning a surface where a residue of water stains, or oxides, or in such instance where soap scum in a shower stall is found, an acid based cleaner, like Sno-Brite Clinging Bowl Cleaner would be used to remove the buildup from the surface. Most of the acids used for cleaning purposes are mild solutions of diluted phosphoric or hydrochloric acid. In some cases a blend of acids might be offered as a cleaning agent since even at very high dilution rates can be quite harmful to human tissue as well as inanimate objects.
Some of the problems faced in the cleaning of hard surfaces and the type of soil problem are indicated below:
Acid Soil Conditions - Requires Alkaline Cleaner
- For a acid soil condition, use a degreaser like; Maxim® ‘Bulldozer’ Cleaner & Degreaser or CleanFreak® 'Eliminator' Heavy Duty Floor Cleaning Degreaser.
- Motor Oils
- Oily Hands
- Diesel Oil
- Engines
- Cooking Oil
- Tools
- Dirty Walls
- Vent-A-Hoods
- Ovens
- Fifth Wheels
- Cigarette Tars
- Axle Grease
- Oil from Skin
- Greasy Floors
If one can determine the basic soil condition, then selection of the correct product for cleaning is much easier.
Alkaline Soil Conditions - Requires Acid Cleaner
- For an alkaline soil condition, use a disinfectant like; Maxim® Facility+ One-Step Disinfectant Cleaner & Deodorant.
- Milk Stone
- Metal Oxides
- Water Spots
- Ferrous Oxide (Rust)
- Calcium Deposits
- Inside of Dishwasher
- Lime Deposits
- Toilet Bowls
- Uric Acid Salts
- Urinals
- Dirty Shower Stalls
There are 3 ways to determine pH.
- Litmus Paper - If acidic, paper turns red. If alkaline, paper turns blue.
- pH Paper - Paper turns various colors depending on the strength of the acid or alkali. This method is the crudest of the three in order to determine pH.
- Electronic pH Meters - Provide an accurate quantitative reading and is a must for research and quality control.
Finally, there is one other factor relative to pH that needs to be explained, and has to do with the corrosiveness of a compound.
Both acids and alkali's have the capability of being corrosive, although one would have a pH range of 0 (acid), while the other would range in the area of 14 (alkali). Sodium hydroxide, a very strong and corrosive alkali would have the same damaging effect on human tissue as sulfuric acid.
If a 25% concentration of sulfuric acid and phosphoric acid were measured for pH, both would range in the area of 0. However, if sulfuric acid were allowed to contact human tissue, severe burns would result, while the average person would not detect even a burning sensation from contact with the phosphoric acid. Why? The answer lies in the corrosive nature of some acids over others.
Through experimentation and testing over the years by chemists, certain characteristics were observed in reactions of acids and alkalis, and were assigned classifications accordingly. One of the classifications is corrosiveness. Therefore to classify a product or compound as being corrosive means that it would have the potential to eat away at something, in some cases very rapidly, and it would have the capability of being harmful to human tissue as well as inanimate objects.
It is incumbent upon each one who offers cleaning products for sale to understand that some compounds are corrosive by their very nature and should be handled according to the label directions on the container.All corrosive products are labeled as such and must follow strict guidelines imposed by the Federal Government.
As one studies proper use of cleaning products, it will become clear that knowledge of pH is a very important issue.

 Protect & Save with SuperFreak.
Protect & Save with SuperFreak.


